







































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Thermofluids Notes on Fluid Statics and Work and Heat.
Typology: Slides
1 / 54

This page cannot be seen from the preview
Don't miss anything!















































G. Mazzanti Process Engineering and Applied Science Dalhousie University Fall 2019 Slides by Michele Hastie, 2016
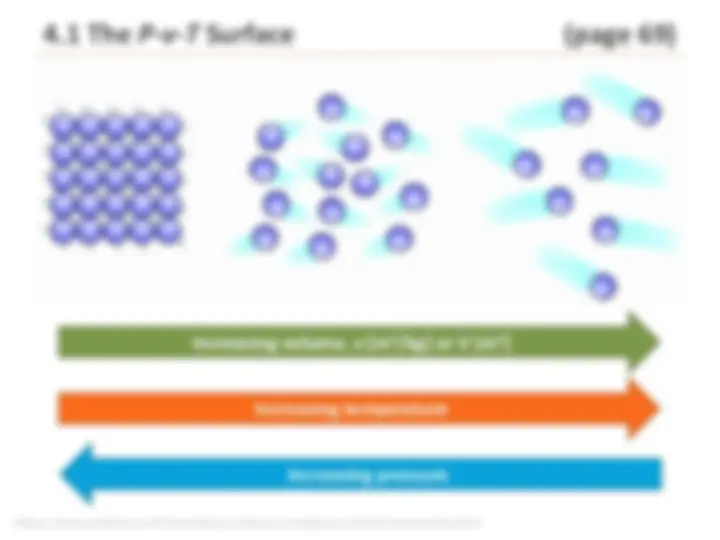
https://www.patana.ac.th/secondary/science/anrophysics/unit5/commentary.htm Increasing temperature Increasing pressure Increasing volume, v [m 3 /kg] or V [m 3 ]
T ν T ν A B C D E F A B C D E F liquid-solid solid-vapour solid liquid liquid- vapour vapour critical point constant P a) b)
Liquid Heat b)
T ν T ν A B C D E F A B C D E F liquid-solid solid-vapour solid liquid liquid- vapour vapour critical point constant P a) b)
Fig. 4.3: P-v-T surface of a substance that contracts on freezing Triple line L-V Solid S-V S-L Liquid Vapour Gas Critical point constant P T = (^) T c
T ν A B C D E F liquid-solid solid-vapour solid liquid liquid- vapour vapour critical point constant P
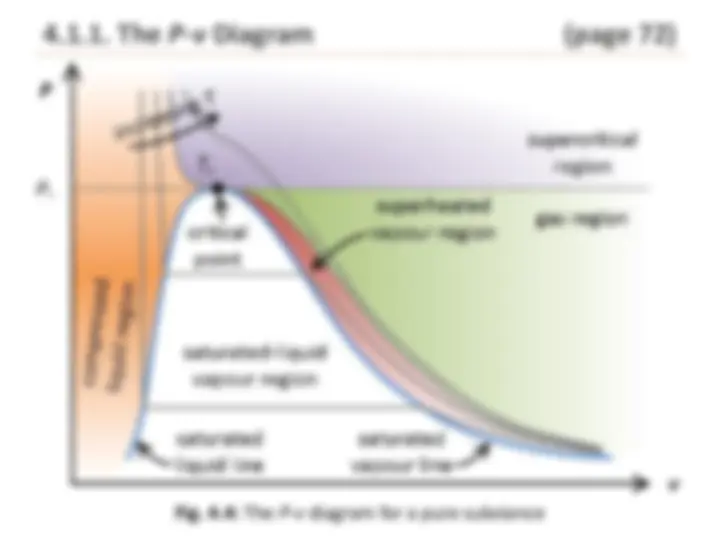
Fig. 4.4: The P - v diagram for a pure substance
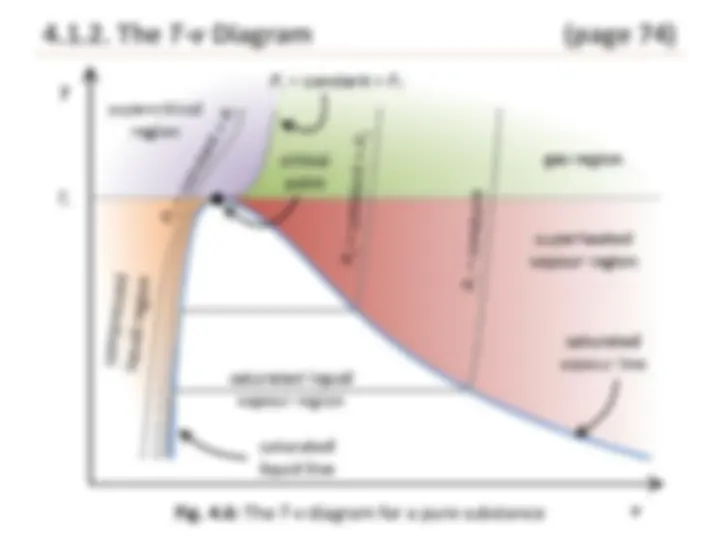
Fig. 4.6: The T - v diagram for a pure substance
A vapour that cannot be condensed simply by increasing the pressure without changing the temperature is normally referred to as a gas. Above the critical point , gas and liquid phases are indistinguishable and the fluid is called a supercritical fluid.
Fig. 4.7: The P - T diagram for a pure substance
Fig. 4.9: T - v diagram showing saturated liquid and vapour points
(4.3b)
The total volume (m 3 ) of the liquid-vapour mixture is,
3
The quality of the mixture is defined as the ratio of the mass of the saturated vapour to the total mass: Quality can be expressed as a fraction (between 0 and 1) or a percentage (between 0 and 100%). V = VL + VV mv = mL vL + mVvV m m x V = (4.3a) (4.4) x = 0 (all saturated liquid) x = 0.5 (50% sat. liquid, 50% sat. vapour by mass) x = 1 (all saturated vapour)
P h critical point Lines of constant specific volume Lines of constant temperature Lines of constant dryness fraction Fig. 4.10: Pressure-enthalpy ( P - h ) diagram
https://www.ohio.edu/mechanical/thermo/property_tables/H2O/ph_water.html
(3.2)
Only intensive variables can be used to specify the state of a system:
Extensive variables cannot be used ( e.g. , mass or volume). Number of phases # of variables Compressed liquid 1 2 – 1 + 1 = 2 Saturated liquid-vapour mixture 2 2 – 2 + 1 = 1 Superheated vapour 1 2 – 1 + 1 = 2