



























Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Thermofluids Notes on Fluid Statics and Work and Heat.
Typology: Slides
1 / 39

This page cannot be seen from the preview
Don't miss anything!
































Outline
3.1 Forms of Energy
3.1.1 Internal Energy
3.1.2 Kinetic Energy
3.1.3 Potential Energy
3.1.4 Mechanical Energy
3.1.5 Nuclear Energy
3.2 Energy Transfer
3.3 Mechanical Forms of Work
3.3.1 Shaft Work
3.3.2 Spring Work
3.3.3 Non-Mechanical Forms of Work
3.4 Temperature and the Zeroth Law of Thermodynamics
3.5 Problems
Introduction (page 56)
Commonly a gravitational or electromagnetic field A fluid under pressure can also be considered a form of potential energy
Molecular motion, electromagnetic interactions, interactions between atomic and subatomic constituents
http://tqees.ie/products-solutions/industrial/ http://www.goodenergy.cl/eng_index.html
Introduction (page 57)
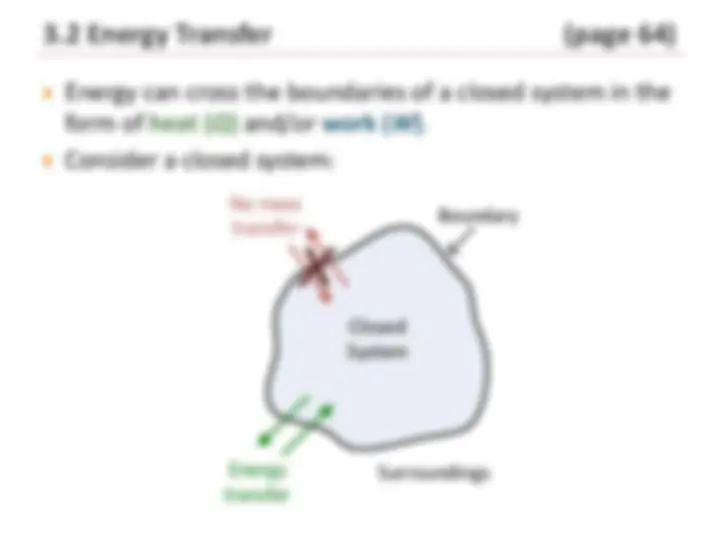
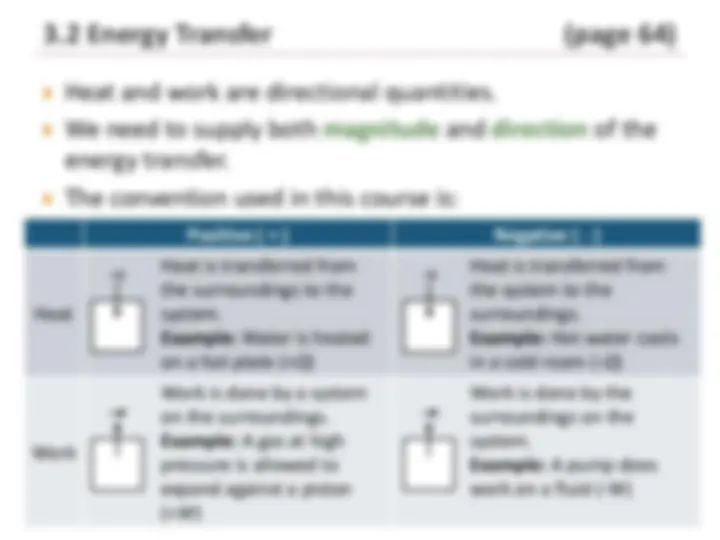
Energy can be transferred between a system and its
surroundings in the form of:
Heat – energy that flows from a high temperature region to a
low-temperature region.
Work – energy that enters or leaves a system as a result of any
driving force other than a temperature difference.
gas
Work
gas gas
Heat
The state of a system is described by its properties at a
particular instant:
Temperature
Pressure
Specific volume
Density
Specific internal energy
Specific heat capacity
etc.
For a single-phase, single-species system, the state can be
fixed by specifying two independent properties.
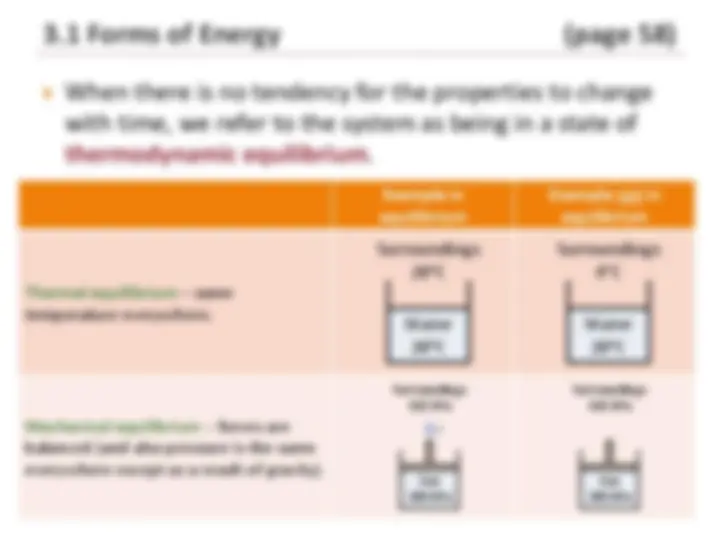
When there is no tendency for the properties to change
with time, we refer to the system as being in a state of
thermodynamic equilibrium.
Example in equilibrium
Example not in equilibrium
Thermal equilibrium – same
temperature everywhere.
Mechanical equilibrium – forces are
balanced (and also pressure is the same
everywhere except as a result of gravity).
Water
20°C
Surroundings
20°C
Water
20°C
Surroundings
4°C
Gas 200 kPa
Surroundings 101 kPa
F
Gas 200 kPa
Surroundings 101 kPa
When a system changes from one equilibrium state to another, the
path of successive states that the system goes through is called a process.
Many real processes can be approximated as an equilibrium process.
If a process is carried out relatively slowly , it will be quasi-static and
so can be considered in quasi-equilibrium.
We may choose to hold a particular property constant
throughout a process
Isothermal – Constant temperature
Isobaric – Constant pressure
Isochoric – Constant volume
If a system undergoes a process that returns to its initial
state, it is said to have undergone a cycle.
3.1.2 Kinetic Energy (KE)
Energy a system possesses by virtue of its motion relative
to some frame of reference.
For a rotating body,
^ (3.3)
= = Nm= J s
m kg 2
1
2
2 2 KE mu
= = kg
J
kg
N m
kg m s
N
s
m
s
m
2
2 - 2
2
2
2 2 u ke (3.4)
= =J s
1 kg m 2
1
2
2 2 KE I ^ (3.5)
3.1.3 Potential Energy (PE)
Energy a system posses by virtue of its position in a
potential field relative to some arbitrary reference point.
Commonly a gravitational or electromagnetic field.
Other forms of potential energy:
Elastic potential energy or “spring energy”.
Chemical potential energy (from chemical bonds or structural
arrangement of molecules).
(3.6)
= m =J s
m PE mgz kg (^2)
= = kg
J m s
m
2
pe gz (3.7)
Closed systems:
Open systems:
where:
( )
E U
E U m u mg z
=
= + + J 2
(^1 ) (3.8)
= kg
J e ˆ^ u^ ˆ (3.9)
0 0
=
kg
J
s
kg
s
J
E m e^ ˆ
m = V = Auavg
Rigid (or fixed) boundary
Movable (or deformable) boundary
gas
Real boundary
Imaginary boundary
Example 3.1: A 2500 kg Jeep travelling at 100 km/h slams
into the back of a stationary 1200 kg automobile. After the
collision, the Jeep slows to 50 km/h and the smaller vehicle
has a speed of 80 km/h. Taking both vehicles as the system,
what is the increase in internal energy of the system?
Solution
Assume that there is no heat transferred to or work done
on the surroundings. The total energy of the vehicles at the
initial conditions must equal the total energy of the
final conditions (Δ E = 0).
A U KE
U KE
E U KE PE
= −
+ =
= + + =
0
0
0
u auto,1 = 0 u jeep, 1 = 100 km/h
u auto,2 = 80 km/h u jeep,2 = 80 km/h
The final kinetic energy is,
The change in internal energy must be equal to the
change in the kinetic energy,
f
KE 2 = KE jeep, 2 + KE auto, 2 = 537. 4 kJ
U = KE 1 − KE 2 = 427. 1 kJ
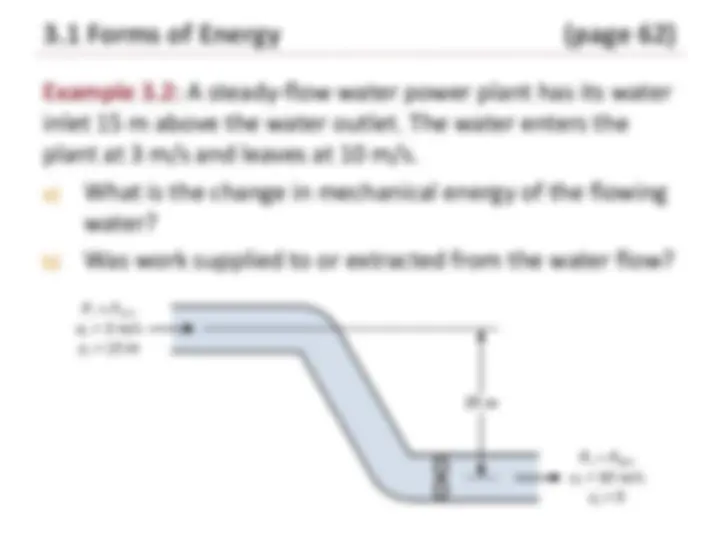
3.1.4 Mechanical Energy
Many practical engineering applications
involve fluid flow through pipe systems
and equipment:
Liquid is pumped up to a higher elevation.
Liquid is allowed to flow under the force of
gravity to turn turbines to generate
electricity.
Air is moved around in a building using
fans.
These examples involve mechanical
forms of energy (pumps, compressors,
fans, turbines).
http://all-flo.com/blog/the-history-of-air-operated-double-diaphragm-pumps-part-two/ http://www.powerpoint.com.my/result.php?root=MTc2&detail=NTQ0&main=MjA0&sub= https://en.wikipedia.org/wiki/Wind_power