




















Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Thermofluids Notes on Fluid Statics and Work and Heat.
Typology: Slides
1 / 28

This page cannot be seen from the preview
Don't miss anything!





















Chapter 2: Fluid Statics
G. Mazzanti
Process Engineering and Applied Science
Dalhousie University
Fall 2019
Slides by Michele Hastie, 2016
Slide 13 (page 31): Added diagram
Added Slide 28
Outline
2.1 Pressure
2.2 Pressure-Elevation Relationships
2.3 Manometers
2.4 Buoyancy and Stability
2.5 Hydrostatic Forces of Submerged Surfaces
2.6 Tank Failures
2.7 Problems
We will not cover
this section.
2.1 Pressure (page 26)
Pressure – the effect of a
normal force acting on an area
When a force Δ F acts at an
angle to an area Δ A it is only
the normal component Δ Fn
that is used to define pressure:
Common units of pressure:
A
F P
n
A (^)
→ 0
lim
2.1 Pressure (page 26)
Relative to pressure in a perfect
vacuum
Relative to atmospheric pressure
Most calculations require absolute
pressure ( e.g ., ideal gas law).
When calculating a change in
pressure, either gauge or absolute
pressure can be used ( e.g .,
pressure-depth equation).
Pabs = Patm + Pgauge
2.2 Pressure-Elevation Relationships (page 28)
Consider a small volume
(Δ x Δ y Δ z ) in a large mass
of fluid at rest.
Since the fluid is at rest, the sum of all the forces must be
zero.
Force balance in the z - direction:
Dividing by Δ x Δ y Δ z :
( Pz (^) = 0 ) x y −( Pz = z ) x y = g x y z
g
z
Pz (^) z Pz = −
= −^ = 0 g s
dz
dP = − = −
Specific weight
2.2 Pressure-Elevation Relationships (page 29)
Patm
P 1
P 2
P 3
Mercury
Water
Pressure is greater in mercury (does not equal P 3 )
2.2 Pressure-Elevation Relationships (page 29)
2.2.1 Constant Density
Pressure can be measured in terms of fluid height.
Invention of the mercury barometer is credited to
Torricelli (1608–1647).
http://ap-physics.david-s.org/simple-mercury-barometer/
kg
m
kg 13595
Pa
N m 101325 Pa
3
2
2 1
−
h
P g h
P P g h
atm^
2.2 Pressure-Elevation Relationships (page 31)
Example 2.1: What pressure would a
diver experience at a depth of 200 ft?
The water may be considered
incompressible with a density of
62.4 lbm/ft
3
. Work entirely in fps units.
c c
g g P P z z h g g
− = − − =
2 1 c
g P P h g
= +
( )
2 m (^2 ) m 2 f
ft
lb (^) s 14.7 psi 62.4 200 ft ft ft lb
lb s
P
= + ^ (^)
2 f (^2 2 )
lb ft 14.7 psi 12480 ft 144 in
P
^ = + (^)
P 2 (^) = 14.7 psi +86.7 psi P 2 (^) =101.4 psia (absolute)
sea level
increasing elevation
u
2.2 Pressure-Elevation Relationships (page 31)
(2.12)
( 2 1 )
1
2 ln z z RT
gM
u
2
1
2
1
P
P
z
u z
dz RT
gM
dP
gM z P P
u
2 1 exp (2.13)
g dz
dP
Note: Density is not
constant because it depends
on pressure.
2.2 Pressure-Elevation Relationships (page 32 )
2.2.3 Isentropic, Perfect Gas (Ideal Gas)
For a perfect gas undergoing an isentropic
process, the pressure-density relationship is:
Note that: and
Therefore:
k k Pv P
− = constant =
k
s
s
P
P
1
0 0 0
= =
=
3
3
m
kg
1
kg
m
v v
p
c
c k =
0 0 =^ =constant
− k − k P P
k
P
P
1
0
(^0)
= (2.14)
Need an
expression for ρ
as a function of P.
2.3 Manometers (page 38)
Manometer – device that uses a column of liquid to
measure pressure.
h
Patm
Pressure
Vessel
( ) 2 1 2 1
P − P = P = − g z − z
P 2 , z 2
P 1 , z 2
1
P gh gx gx P atm
2.3 Manometers (page 39)
P 1 = Patm + gh
atm m o
P P gh gh
P P gh gh gh
= + − −
= + − −
3
3
P P ( ) gh
P P gh gh gh gh
m
o m o
= + −
= + + − −
5 4
5 4
atm m o
P P gh gh
P P gh gh gh
= + − −
= + − −
6
6
P 2 = Patm + gh
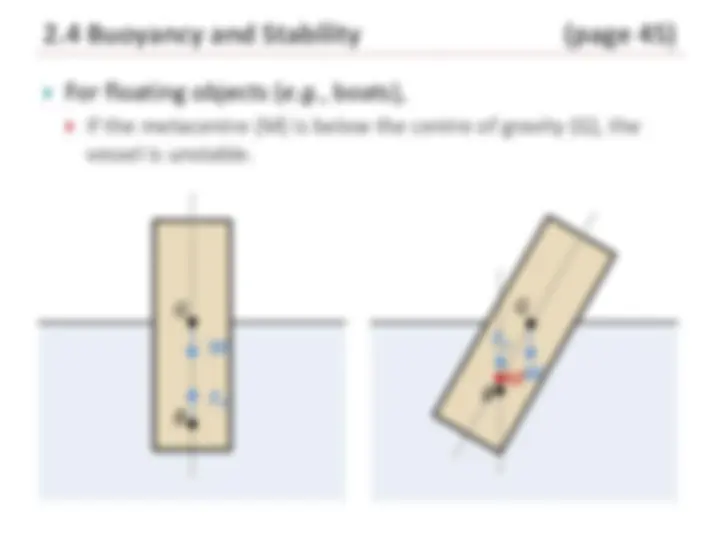
2.4 Buoyancy and Stability (page 41 )
Archimedes Principle:
For an object to remain at rest within the fluid (neutrally
buoyant), the weight ( W ) must be balanced by the
buoyant force:
f displaced
W = gV
Fabove
Fbelow
Fabove = Weight from fluid above
Fbelow Fbelow = Weight from fluid above
Fabove
Fabove
Fbelow
Fabove = Weight from fluid above
Fbelow Fbelow = Weight from fluid above
Fabove
2.4 Buoyancy and Stability (page 41)
Archimedes Principle:
For an object to remain at rest at the surface, the weight
of an object ( W ) must be balanced by the buoyant force:
Only weight of liquid
is considered
because ρ liquid >> ρ gas
f displaced
W = gV