














Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Thermofluids Notes on Fluid Statics and Work and Heat.
Typology: Slides
1 / 25

This page cannot be seen from the preview
Don't miss anything!


















ENGI 2102 Thermo-Fluid Engineering I
G. Mazzanti Process Engineering and Applied Science Dalhousie University Fall 2019 Slides by Michele Hastie, 2016
1.1 Systems and Control Volumes
1.2 Properties of a System
1.3 Density and Specific Gravity
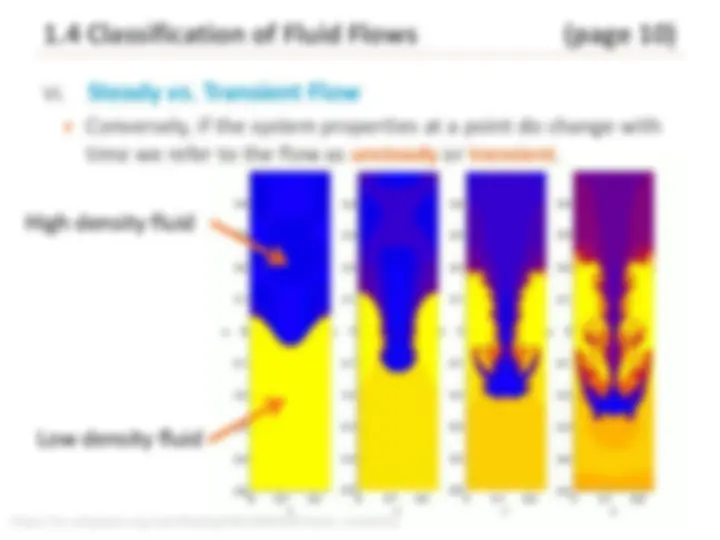
1.4 Classification of Fluid Flows
1.5 Viscosity
1.6 Surface Tension and Capillary Effects
1.7 Problems
We will return to this material at the end of the semester.
A real boundary may be either rigid or deformable (Fig 1.1b)
Closed system (Fig. 1.1c)
Mass (fluid) does not cross the system boundary Energy can cross the system boundary
b) A system with rigid and deformable
(or deformable)Movable Rigid (or fixed)boundary boundary
gas
boundaries
c) A closed system or control mass
SystemClosed
Boundary
Energy Surroundings transfer
No masstransfer
Open system (Fig 1.1d) – Both mass (fluid) and energy can cross the system boundary
Isolated system – Neither mass nor energy crosses the system boundary
d) An open system or control volume
Imaginaryboundary boundaryReal
Extensive property – dependent on the quantity (or extent) of matter in the system Mass and volume are extensive properties Extensive properties are additive
10 kg of water 2 m 3 of air
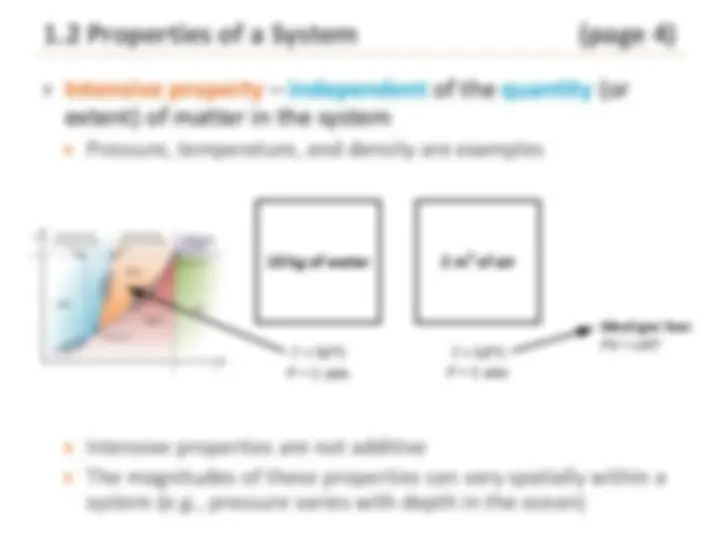
Intensive property – independent of the quantity (or extent) of matter in the system Pressure, temperature, and density are examples
Intensive properties are not additive The magnitudes of these properties can vary spatially within a system ( e.g. , pressure varies with depth in the ocean)
10 kg of water 2 m 3 of air Gas
SupercriticalFluid
Melting/Solidification
P
T
Critical Point
Sublimation/Deposition Triple Point
expand on freezingSubstances that contract on freezingSubstances that
T (^) c
Pc
Vaporization/Condensation Vapour
Liquid Solid
T = 50°C P = 1 atm
T = 50°C P = 1 atm
Ideal gas law: PV = nRT
Non-equilibrium thermodynamics: (rate processes, transport phenomena, heat and mass transfer): The time course of the process is considered.
Example: How can we design a device to quickly heat liquid water from 20 to 100°C? To answer this question we need to consider: The power of the heat source The conductivity of the material involved Is the water being stirred or is it stagnant? http://www.wholeheartedplumbing.com/unclog-drain-boiling-water/
We can analyze a system from two different points of view: Microscopic viewpoint: Considers the behaviour of every molecule Microscopic properties: Molecular diameter, mean free path, kinetic energy of individual molecules, etc.
Macroscopic viewpoint: Concerned with the average effects of many molecules’ interactions Macroscopic properties: Pressure, temperature, density, etc.
For most engineering systems we are typically only concerned with macroscopic properties and their variation with space and time.
Example: Pressure (macroscopic) is the force per unit area that molecules (microscopic) exert on container walls as the collide with it.
Statistical mechanics, statistical thermodynamics
Continuum mechanics, classical mechanics, classical thermodynamics
A fluid is a form of matter that is unable to withstand an applied shear stress ( τ ). Given sufficient time, the smallest shear stress is capable of producing any change of shape. This is in contrast to the behaviour of a solid , in which a definite value of the shearing stress must be applied to produce a given deformation.
Solid
Fluid
τ τ τ
τ τ τ
Time τ
τ
A fluid offers no resistance to change of shape. But it does exhibit a resistance to the rate of change of shape. Viscosity – a measure of a fluid’s resistance to gradual deformation by shear stress
http://www.laboratoryequipment.com/news/2012/01/liquid-more-liquid-water
Fluid flow can be classified in a number of ways:
I. Viscous vs****. Inviscid
Flows where fluid friction effects are significant are called viscous flows Conversely, flows where fluid friction is negligible are termed inviscid flows
II. Internal vs****. External Flow
Flow is classified as internal if it flows within a confined space (pipe or duct).
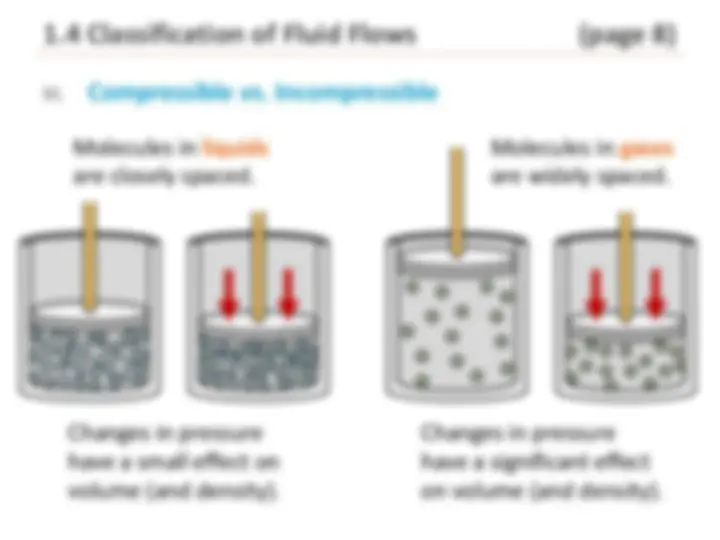
III. Compressible vs****. Incompressible
Molecules in liquids are closely spaced.
Changes in pressure have a small effect on volume (and density).
Molecules in gases are widely spaced.
Changes in pressure have a significant effect on volume (and density).
III. Compressible vs****. Incompressible
Compressibility is a measure of the volume (or density) change of a fluid (or solid) as a response to pressure. Compressible flow – Density changes along streamlines Incompressible flow – Density variations along streamlines are negligible More variables and equations are required to describe compressible flows. In general:
Incompressible Flow Compressible Flow Liquids (most situations) Gases at low velocities
Gases at high velocities Some situations involving liquids