



Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
The role of the secondary structure of the iron-responsive element (IRE) in the translational regulation of ferritin synthesis. The authors investigate the effects of mutations in the IRE on its ability to regulate translation in response to iron. They find that only small perturbations in the affinity of interaction between the iron regulatory protein (IRP) and the IRE result in a loss of iron-dependent translation of ferritin mRNA. The document also discusses the importance of the IRE in coordinating the synthesis of proteins involved in iron uptake, storage, and utilization.
What you will learn
Typology: Lecture notes
1 / 6

This page cannot be seen from the preview
Don't miss anything!




Role of RNA secondary structure of the
iron responsive element in translational regulation of
ferritin synthesis
Zora (^) KikInisI,2,*, Richard S. Eisenstein1'2'+, Andrew J. E. Bettany"p§ and Hamish N. Munro19Z
Received May 18, 1995; Revised and Accepted August 29, 1995
between a stem-loop structure, the iron-responsive element (IRE), located in^ the^ 5'-untranslated^ region (5'-UTR) of^ ferrMn mRNAs, and^ a^ protein, the^ Iron regulatory protein (IRP). The^ role^ of^ IRE^ secondary structure in^ translational regulation of^ ferritin^ syn- thesis was explored by Introducing ferritin^ constructs containing mutations^ in^ the^ IRE into^ Rat-2^ fibroblasts. Our In vivo studies demonstrate that size and sequence of the^ loop within the^ IRE^ and the^ distance and/or (^) spatial relationship of this (^) loop to the (^) bulged
served in order to observe iron-dependent translation of ferritin mRNA. In contrast, changes in nucleotide
affectIng translational regulation in vivo, as long as a stem can be formed. Our in vivo results suggest that
of IRP with IRE can be tolerated in order to maintain iron-dependent regulation of translation.
tional levels (^) (7). At low iron levels translation of both subunit mRNAs is inhibited and the mRNAs are present in the translationally inactive ribonucleoprotein (RNP) form. When the intracellular level of iron is raised these dormant mRNAs are activated and become associated with polysomes (8-10).
The mechanism for translational regulation offerritin synthesis involves the 5'-untranslated region (5'-UTR) of ferritin mRNAs, where a sequence of 28 nt is almost perfectly preserved in H- and
sequence, which has been named the iron-responsive element (IRE), is essential for regulation of ferritin mRNA translation by iron (11-13). A specific cytoplasmic protein, iron regulatory protein (IRP; formerly called IRE binding protein, IRE-BP, iron
binds to the IRE (^) (14,15) and represses translation of ferritin
in initiation of mRNA translation, apparently through regulation of the binding of the^ 43S pre-initiation complex to^ the mRNA (17-21). IREs have been found in the^ mRNAs^ of proteins involved^ in other aspects of iron metabolism or other cellular functions. In
drial aconitase mRNA (25). In contrast to the single IRE located
iron-dependent regulation of TfR mRNA stability (22,23).
utilization of iron. Several studies have used a structural or a functional approach to understand the function ofthe IRE. The IRE forms a stem-loop
consists of a long stem interrupted at several sites by bulged regions and a six membered loop of CAGUGX, in which X can be A but is usually U or C (26). Recent evidence suggests a base
*To whom correspondence should be addressed at: Genetics Laboratory, HNRCA at Tufts University, 711 Washington Street, Boston, MA 02111, USA Present addresses: (^) +Department of Nutritional (^) Sciences, University of (^) Wisconsin, 1415 Linden (^) Drive, Madison, WI 53706, USA and lnstitute of Grassland and Environmental (^) Research, Plas (^) Gogerddan, Aberystwyth, Dyfed SY23 3EB, UK 1Deceased
raising the possibility that the loop may be 3 nt in size with a bulged nucleotide on the 3'-side of this smaller loop (30,33). However, based on accessibility to single-strand-specific nucleases it appears that the loop is 6 nt in size at least part of the time (29). The requirement for the secondary structure has been tested in experiments with mutant IREs, which were analyzed either for their (^) binding to the IRP in vitro or for their ability to regulate translation in vivo. Substitution of nucleotides in the loop ( 1 5,28,31-33), disruption of base pairing in the (^) upper part of the stem (29,34) and (^) replacement of the bulged C (31-33) decreased the binding affinity of the IRP for the mutant IREs in (^) almost all cases. Mutant IREs with nucleotides deleted either from the (^) loop or the upper stem failed to regulate translation in (^) response to iron in vitro or in vivo (15,18,19,26). While these studies examined the binding affinities and/or the in vivo function ofvarious (^) IREs, none of these mutants have been analyzed simultaneously for their RNA secondary structure, binding affinity to the IRP and functionality in vivo. In order to identify functional elements of the stem-loop structure of the IRE, mutants with changes in both secondary structure and primary sequence were designed (Fig. 1) and tested for function as regulators of translation in response to iron. These mutant IREs were inserted into a ferritin reporter gene and tested for their ability to regulate iron-dependent translation of the reporter gene in rat fibroblasts. This approach enabled us to determine the relationship between formation of base pairs in the upper stem, the length of the stem, the size and the sequence of the loop and the ability of the IRE to function in vivo. Since our previous work determined the secondary structure of these variants by RNase mapping, as well the effect of these mutations on the (^) binding affinity of IRP (^) using quantitative band-shift assays (29), we can now relate (^) these data to function in vivo. Taken together our^ results^ indicate that only small perturbations in the affinity of interaction of IRP^ with the IRE result in a loss of iron-dependent translation of ferritin (^) mRNA.
G U A G C (^) A-UU A-U C-G U G U-A CG-C 40- U G -
A^ G U G C (^) A UU A A C U U U U C CG-C 40- U-A -
AG U G C (^) A UU U U G G AG GA C G-C 40- U-A -
G U A G C (^) A-UU U-A G U A-U G-C CG-C 40- U-A -
wild (^) type 3'stes mutated 5'stes mutated stem restored
G U A G C U A C A-U A-UC-G U G U-A C G-C 40- U G -
G U A G C U G-C A-U A-UC-G U G U-A C G-C 40- U G -
U G G A U C A-UA-U C-G U G U-A C G-C 40- U G -
large loop long stem reversed loop
Figure 1. Nucleotide sequence and (^) secondary structure of the wild-type and mutant IREs. The naturally occurring IRE sequence (nucleotide (^) positions 40-60 of the L-ferritin cDNA clone (^) indicated) folds into a stem-loop structure and is presented on the far left side. The six mutants follow. (^) Nucleotides substituted or inserted are indicated in bold type and the secondary (^) structure of the variant IRE is presented schematically.
A
5'UTR protein coding region (^) 3'UTR
+1 34 (^59 210 687 710 850) 900bp
Plasmid constructs
As a reporter gene we used a rat L-ferritin (35,36) cDNA from which the 24 nt (^) encoding the octapeptide sequence in the protein were deleted. The deletion (^) was performed using the Muta-gene M13 in vitro (^) mutagenesis system (BioRad). Briefly, a 244 bp NcoI-StuI (^) fragment was (^) excised from the rat L-ferritin full-length cDNA clone (^) pl6Bgl (37) and (^) subcloned into M13mpl8, which allows (^) synthesis of (^) single-stranded DNA. A (^) 60mer oligonucleo- tide, spanning 30 nt on^ each side of the^ octapeptide sequence, was used as (^) mutagenic primer and annealed to the M13 DNA. For further steps we followed the manufacturer's (^) protocol. Finally, the NcoI-StuI fragment carrying the deletion was resubcloned into pl6Bgl. The L-ferritin sequence deprived of the (^) octapeptide region was designated shortened ferritin (Fig. 2). When this shortened ferritin was expressed in rat fibroblasts it migrated below the endogenous L-ferritin protein on SDS-PAGE gels, thereby
The variant IREs have been described (^) previously (29).
generated by replacing the IRE from the natural 5'-UTR region with the mutated IRE^ as AvaI-AvaI fragments. The construction
B
5'UTR protein coding region 3'UTR
+1 34 (^59 210 686711 850) 900bp
Figure 2. L-Ferritin cDNA clone and shortened ferritin construct. (A) The full-length rat L-ferritin cDNA clone is 0.9 kbp long, including the 5'-UTR, protein coding region and the 3'-UTR. Nucleotide position +1 is the transcription start.^ The^ iron-responsive element (IRE) is located at nt 34-59. (B) The^ rat^ L-ferritin octapeptide coding sequence, positions 687-710, has been deleted in the shortened ferritin construct.
(Fig. 1). In addition, a cDNA lacking the IRE (del IRE), was made by excising the IRE sequence between the two AvaI restriction sites (nt 24-68 removed). The various shortened ferritin con- structs were subcloned into the (^) eukaryotic expression vector p (kindly provided by B. Sugden and T. (^) Middleton, McCardle Laboratories, University of Wisconsin). In this vector (^) transcrip- tion was directed from the cytomegalovirus (CMV) promoter and
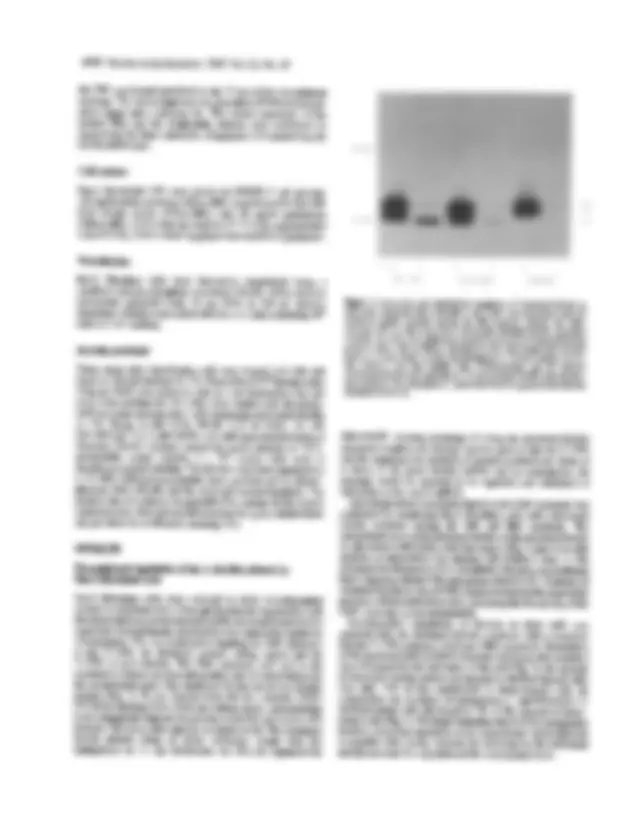
Figure 4. Synthesis of^ shortened^ ferritin^ from^ constructs^ with^ substituted nucleotides in^ the^ stem^ of^ the IRE. Rat-2 fibroblast cells^ were^ transiently transfected with^ a^ shortened^ ferritin^ construct^ carrying^ mutated^ nucleotides^ on the 3'- or 5'-strand^ of^ the^ stem^ and^ finally^ with restored^ stem structure^ of^ the IRE. The^ cells^ were^ treated with^100 gM heme^ (+)^ or^100 gM^ desferal^ (-)^ and synthesis of shortened ferritin (S) from the constructs and ofendogenous ferritin proteins (F)^ was^ assayed^ as^ described^ in^ Figure^ 3.
Effects of base pairing capacity of^ the^ upper^ stem^ on IRE function
We investigated^ the^ relationship^ between the^ secondary^ structure of the IRE and its ability to regulate translation^ using a^ series^ of altered IRE sequences (Fig. 1). The first series^ of^ mutants^ focused on the stem of the stem-loop structure. In^ the^ 3'- and^ 5'-stem^ IRE mutants the upper stem region was^ disrupted^ by changing^4 nt^ on the 3'- or 5'-side of the stem in such a^ way that^ the^ stem^ could^ no longer form. However, since the^ 5'- and^ 3'-stem^ mutants^ were complementary to each other, combination^ of^ the^ new^ 3'- and 5'-stem nucleotides led to a mutant with^ a^ restored^ stem^ structure. Although the primary sequence is^ different^ from the wild-type IRE sequence, the restored stem^ IRE^ mutant^ was^ designed^ to^ give a similar free energy of stem formation^ to^ that ofthe^ wild-type^ and formation of the stem was^ confirmed^ by^ RNase^ mapping^ (29). These mutant IRE constructs^ were^ transfected^ into^ Rat- fibroblast cells and analyzed for^ accumulation^ of^ shortened ferritin in response to^ high or^ low iron^ concentrations.^ In^ the^ case of the 3'- and 5'-stem^ mutant^ IRE^ constructs^ synthesis^ of^ the shortened ferritin was^ independent of^ the^ iron^ status^ of^ the cell (Fig. 4). Thus IRE^ mutants^ with^ a^ disrupted^ stem^ structure^ fail^ to function as^ iron-responsive elements.^ In^ contrast,^ the^ mutant^ IRE with the restored^ stem,^ but^ different^ primary^ sequence^ from^ the wild-type IRE, exhibited^ restoration^ of^ iron-dependent^ synthesis of the shortened ferritin protein.
Stem (^) length, as well as the size and the sequence of^ the loop, affect IRE function
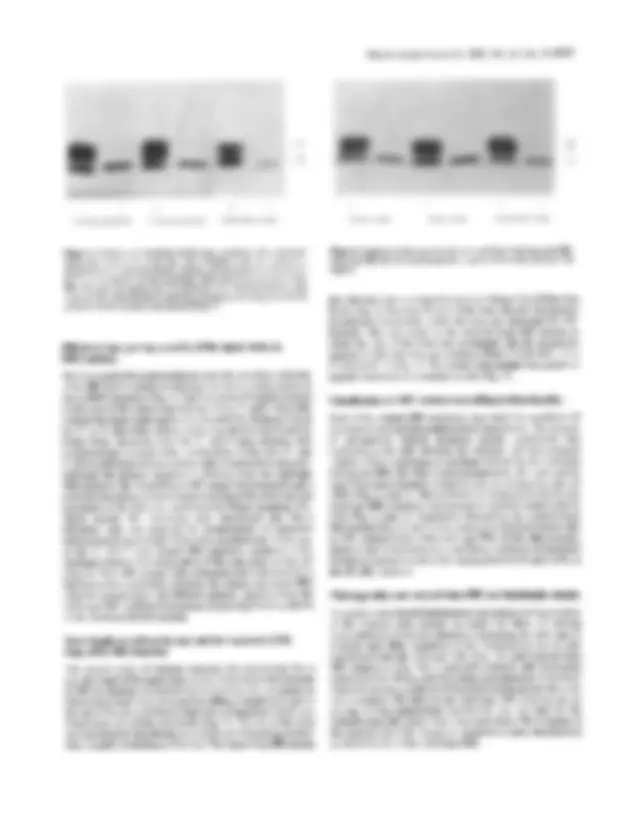
The second^ series of^ mutants^ assessed^ the^ requirement^ for^ a specific length^ ofthe^ upper^ stem^ or^ size^ of^ the^ loop^ on^ the^ function of IRE as mediator of translational control^ in^ vivo.^ A^ mutant^ in which stem length was^ increased^ by adding^ a^ single^ G-C^ pair^ to the top of the^ stem^ exhibited^ a^ high^ rate^ of^ translation^ which^ was independent of cellular^ iron^ levels^ (Fig. 5).^ The size^ of^ the^ loop was increased by introducing two additional non-pairing nucleo- tides, A and C, to the base ofthe loop. This^ larger loop^ IRE^ mutant
large loop long stem reversed^ loop
Figure 5. Synthesis of shortened ferritin from^ constructs^ with long^ stem^ IRE, large loop IRE and a reversed nucleotide sequence^ in^ the^ loop (see Fig. 3 for legend).
also showed a loss of responsiveness to^ changes^ in^ cellular^ iron levels (Fig. 5). Not only the^ size^ of^ the^ loop, but also^ the^ position of particular nucleotides within^ the^ loop,^ are^ important^ for^ IRE function. This was^ tested^ in^ the^ reversed loop^ IRE^ mutant^ in which the size of the loop^ was^ unchanged,^ but^ the^ nucleotide sequence within the^ loop^ was^ reversed (from^ 5'-CAGUGU-3'^ to 5'-UGUGAC-3'; Fig. 1).^ The^ reverse^ loop^ mutant^ was^ unable^ to regulate translation in^ response^ to^ iron^ (Fig.^ 5).
Classification of IRE^ mutants^ according^ to^ functionality
Each of the mutant IRE constructs was tested for^ regulation^ of translation in at least three independent transfections. The^ amount of radioactively labeled shortened ferritin synthesized^ was expressed as the ratio between the desferal- and^ heme-treated samples. Since expression of shortened ferritin by the^ construct lacking the IRE (del IRE) is iron-independent, this^ was^ used^ to represent a non-responsive construct and was^ assigned^ a^ value^ of 100% (Fig. 6, lane 1). The synthesis of^ shortened ferritin^ by the wild-type IRE construct was reduced in^ desferal-treated^ cells^ to 13% (Fig. 6, lane 2). Translation directed by^ the^ restored^ stem IRE mutant (Fig. 6, lane 5) was^ reduced^ in^ desferal-treated^ cells to 19%, similar to that of the^ wild-type IRE. All^ the^ other^ mutants failed to show iron-responsive translation,^ synthesis^ of^ shortened ferritin in desferal-treated cells^ ranging from^ to^90 and^ 115%^ of the del IRE construct.
Wild-type IRE and restored^ stem^ IRE^ are^ functionally^ similar
To (^) acquire more detailed information concerning the functionality of the restored stem mutant we tested the effect of^ varying concentrations of iron on constructs containing the wild-type or restored stem IREs. Synthesis of the shortened^ ferritin in^ cells transfected with the wild-type IRE^ (Fig. 7A)^ and^ restored^ stem IRE constructs (Fig. 7B) is^ gradually reduced^ with^ decreasing concentrations of heme^ and^ increasing^ concentrations^ of^ desferal. Figure 8 compares synthesis^ of^ shortened^ ferritin^ protein^ from the two constructs. The data for the wild-type IRE construct^ are^ an average of two independent transfection sets; the^ data for the restored stem IRE derive from^ one^ experiment.^ The^ response^ of the restored stem IRE^ mutant^ to^ variations^ in^ iron^ concentration is similar to^ that^ of^ the^ wild-type^ IRE.
jml..
1 2 3 4 5 6 7 8
40 20
+100 .50 0 50 100
Figure 6. Translational repression of shortened^ feffitin from^ mutant^ IRE constructs. The translational repression is shown as the^ ratio^ ofprotein synthesis measured in^ cells that have been^ treated with 100^ pM heme^ or^100 IM desferal. Synthesis of shortened ferritin in^ the del IRE^ construct^ (1)^ is^ iron-independent and has been set as^ 100%.^ Translation of the^ wild-type IRE^ construct^ (2)^ is iron-dependent; in^ desferal-treated cells^ only 13% of^ shortened ferritin^ was synthesized. Translational^ repression has been calculated for all^ mutant^ IRE constructs: 3'-stem^ mutant^ (3), 5'-stem mutant^ (4),^ restored^ stem mutant^ (5), large loop mutant^ (6), long^ stem mutant^ (7)^ and reversed^ loop^ mutant^ (8).^ The data reflect the^ average of^ at^ least^ three^ experiments.
..
l 2 iJ.
F
1
.. -~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
IIIm.m~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~. iniuompuomummm
F
1 2 4 b
Figr 7. (^) Dose-response curve. Rat-2 fibroblast cells were transiently tansfected with the shortened ferritin construct of wild-type IRE (A) and restored stem IRE (B). Synthesis of the shortened feritin (S) and the endogenous ferritins^ (F)^ is^ shown^ as a^ response^ to^ decreasing^ iron^ levels in^ cells treated wvith 50 (1), 12.5 (2) or 5 pM heme (3), medium (4) or 5 (5) or 27.5 pM desferal (6).
The iron-dependent synthesis offerritin^ is^ regulated at^ two^ levels, translation (^) and transcription (7). We were able to analyze regulation at the translational level through the use of a recombinant femtin construct in which the complete 5'-UTR, the shortened protein coding region and the 3'-UTR of rat L-ferritin
promoter. The^ synthesis of^ the^ shortened^ ferritin^ L-subunit^ was compared in^ Rat-2^ fibroblast^ cells^ exposed to^ heme^ (high intracellular iron concentration) or desferal (low intracellular iron levels). Using the shortened ferritin construct translation was repressed 90% in desferal-treated cells (Fig. 3). In^ the^ same^ cells synthesis of the endogenous ferritins was^ monitored^ and shown to decrease 98% in^ desferal-treated cells.^ This^ higher range of
Figure 8. Dose-response curve. The expression of shortened ferritin from a construct of (^) wild-type IRE (x) and restored stem IRE (squares) were plotted versus decreasing iron levels in cells. The sample with highest synthesis has been set to 100% and all the other samples are expressed as a percentage of this value.
repression in^ synthesis of^ endogenous ferritin^ presumably represents additional regulation of ferritin by iron^ at^ levels^ other than translation, possibly a^ 5-fold^ regulation^ at^ the^ transcriptional level. Indeed, a 3-fold increase^ in^ transcription^ of^ the L-ferritin gene in^ rat liver cells (7) and^ a^ 4-fold increase^ in^ HeLa^ cells^ (44) has been^ reported.^ Taken^ together^ our^ results^ demonstrate iron-dependent regulation^ of^ ferritin^ mRNA^ translation^ in^ Rat- fibroblast cells and show that the largest contributor to this response is^ alterations^ in^ mRNA^ translation^ rates. Iron-dependent regulation of translation is conferred by the stem-loop structure^ of^ the^ IRE. We^ explored^ this^ relationship with respect to the structural requirement for the stem-loop structure using mutants with altered secondary structure (Fig. 1). Attention has been paid to the correct folding ofthose mutants and their secondary structure was confirmed previously by RNase mapping (29), except for the reverse loop IRE mutant, which was analyzed for secondary structure by Zucker's sub-optimal folding program (A. J. E. Bettany, personal communication). The importance of the formation of the stem was assessed by using two mutant IREs in which the stem structure was disrupted and a third mutant in which the stem was reformed. Both of the^ IRE mutants with disrupted stems failed to respond to low cellular iron levels, whereas the restored stem IRE mutant repressed transla- tion in cells deprived of iron to levels similar to that^ of^ the wild-type IRE (Fig. 4). The response of the^ restored^ stem^ IRE mutant was studied in^ more^ detail. We^ performed a^ detailed analysis of how the^ constructs^ containing the^ restored^ stem^ IRE or (^) wild-type IRE (^) responded to variations in iron concentration. Both IREs (^) responded in an (^) identical manner over a broad range of iron concentrations (^) (Fig. 8). Since the two constructs contain IREs of similar secondary structure and similar free energy of stem formation, but dissimilar primary sequence, we conclude that the presence of a base paired stem is both necessary and sufficient to satisfy the requirements for a^ functional IRE.^ This observation is^ supported by the^ IREs^ found in^ transferrin^ receptor and eALAS mRNAs, where^ the^ nucleotide^ sequences vary,^ but the (^) ability to form base (^) pairs may be preserved (23-25). On the other hand, IREs found in various ferritin mRNAs show a^ striking conservation of the nucleotides in^ the^ stem, which^ cannot^ be explained so^ far.^ From^ our^ data^ we^ have^ to^ conclude^ that iron-dependent repression of^ translation^ does^ not^ require^ the conservation of the stem nucleotides. We also observed that increasing the length of the stem eliminated iron-dependent regulation. The length ofthe stem is an
160 140 120 100 ae 80 60 40 20 0