
























Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An overview of a lecture series on thermodynamics and heat engines. The series covers various topics including motors, generators, distribution and use of electricity, wind energy, thermodynamics, heat engines and transportation, nuclear generation, solar power, and fuel cells. The document also includes resources for further study and explanations of key concepts such as entropy and the second law of thermodynamics.
Typology: Slides
1 / 44

This page cannot be seen from the preview
Don't miss anything!





































C O M P T O N L E C T U R E 6 : N O V E M B E R 7 , 2 0 0 9 E R I C S W I T Z E R
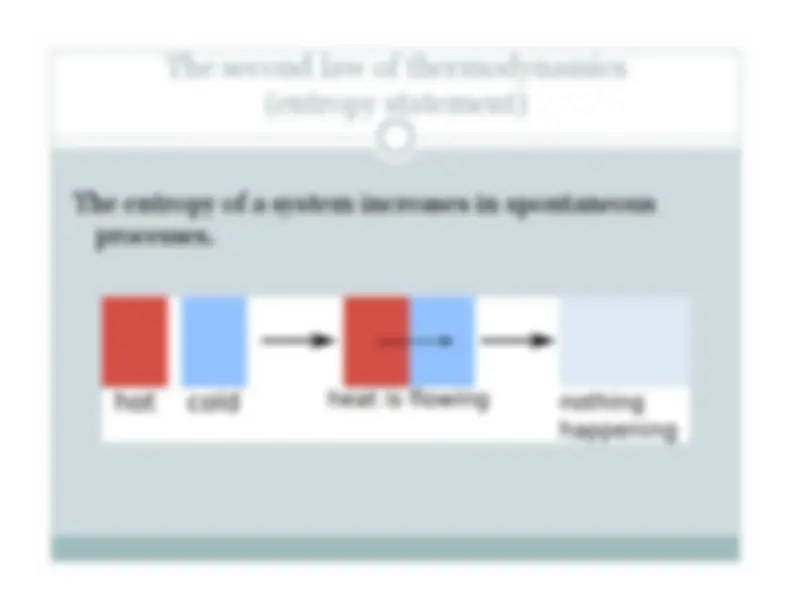
“The study of these engines is of the greatest interest, their importance is enormous, their use is continually increasing, and they seem destined to produce a great revolution in the civilized world.” – S. Carnot 1824 Réflexions sur la puissance motrice du feu
Steam engines Gas/Diesel engines Magnetohydrodynamic generators Thermoelectrics, Thermionics Solar thermal Refrigerators, AC, etc. Perhaps the greatest reward: the drinking bird … Image: wikipedia
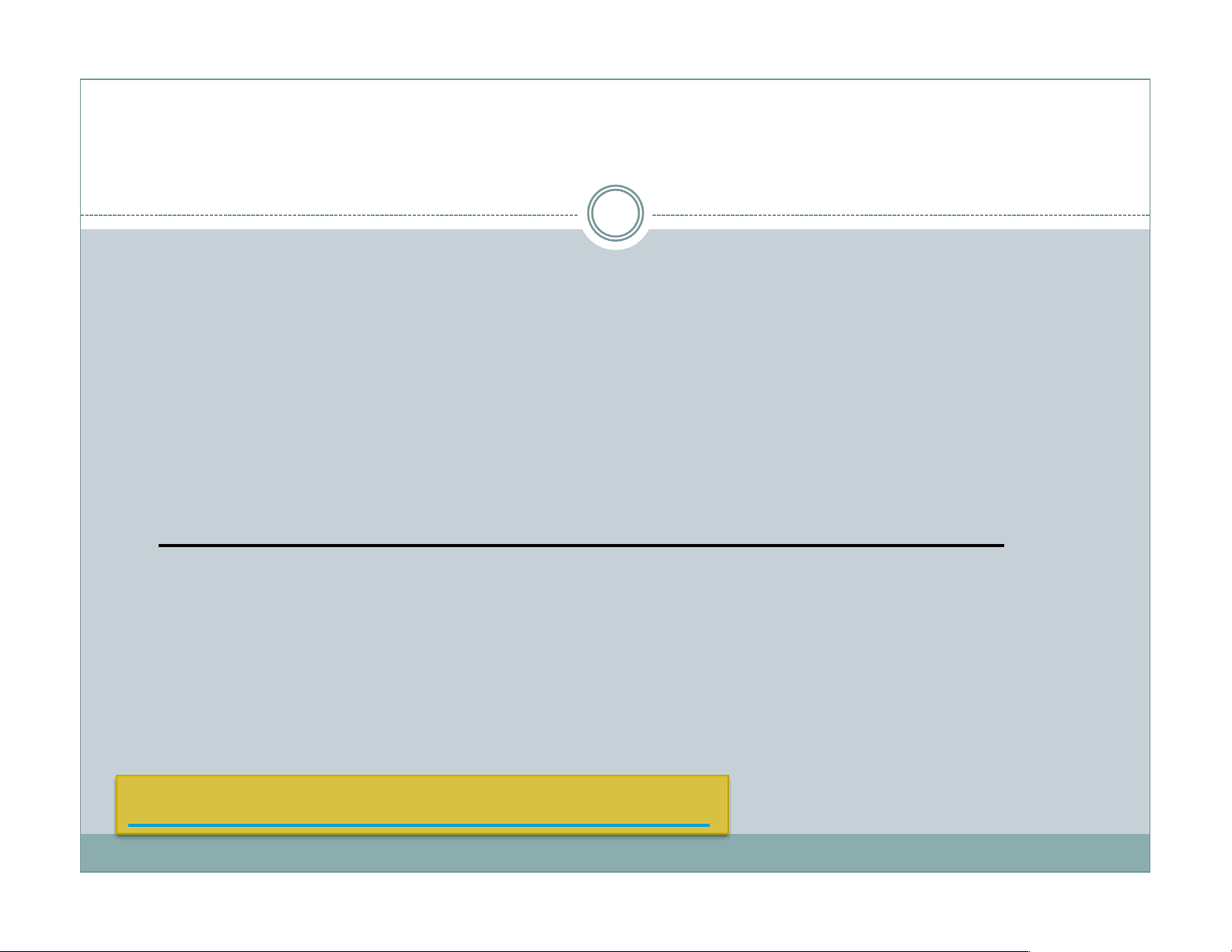
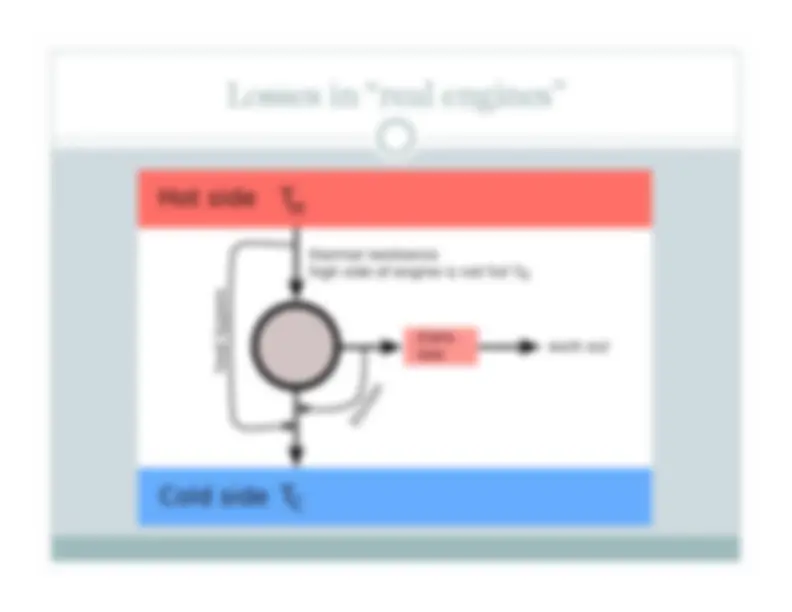
Heat flows across a gradient spontaneously: entropy increases Hot room (298 K), cold ice (273 K) Thermal gradient drives a heat flow Thermal energy ΔQ spread in the cooler system Heat flow continues until the temperatures match (entropy is maximized.) Image: wikipedia Hot Cold Entropy increasing Entropy decreasing Entropy is (globally) increasing.
Heat flows across a gradient spontaneously: entropy increases Image: wikipedia Hot Cold ΔSice = ΔQ/(273 K) ΔSroom = - ΔQ/(298 K) ΔStot= ΔSroom +ΔSice= ΔQ[1/273 – 1/298] > 0* Entropy of the water/ice increases more than the entropy of the room. Thus, a net entropy increase. ΔS = ΔQ/T
Energy is conserved – entropy is not. Entropy “increase” is entropy production Image: wikipedia
x
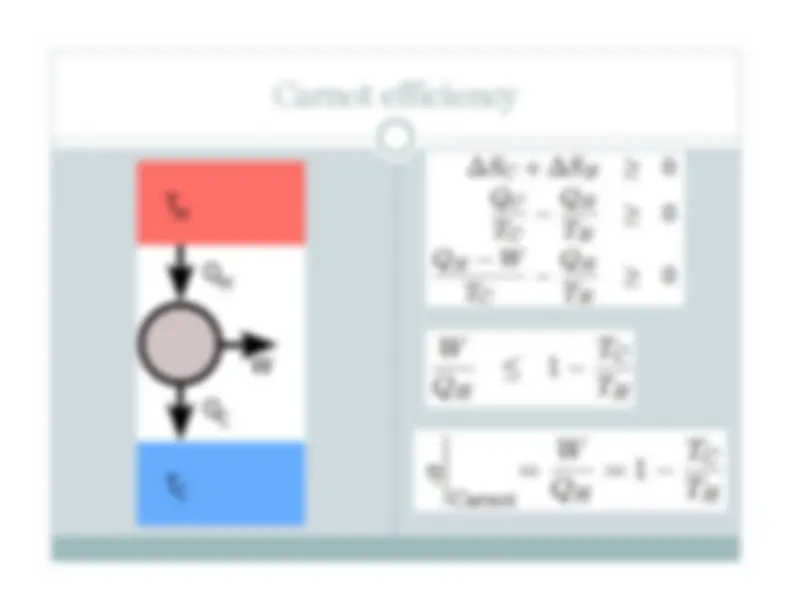
Engines often work over a limited temperature range. So… combine two of them! Efficiency: η = η A +η B
Mitsubishi Heavy Industries M701G outputs 334 MW at 39. percent efficiency, T H
o C (2, o F), T C
o C (1, o F). Using the exhaust in the second cycle, on achieves ~60% efficiency! More exotic: MHD and steam. Images: wikipedia, data “Efficiency by the Numbers” by L. Langston (AMSE)