




Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Carboxylic Acids, Esters, Amines, and Amides
Typology: Exams
1 / 6

This page cannot be seen from the preview
Don't miss anything!




Name: ________________________________
Encircle the letter of the correct answer.
Find A & B in the given reaction a) (CH 3 ) 2 C(OH)CN, (CH 3 ) 2 C(OH)COOH b) (CH 3 ) 2 C(OH)CN, (CH 3 ) 2 C(OH) 2 c) (CH 3 ) 2 C(OH)CN, (CH 3 ) 2 CHCOOH d) (CH 3 ) 2 C(OH)CN, (CH 3 ) 2 C=O
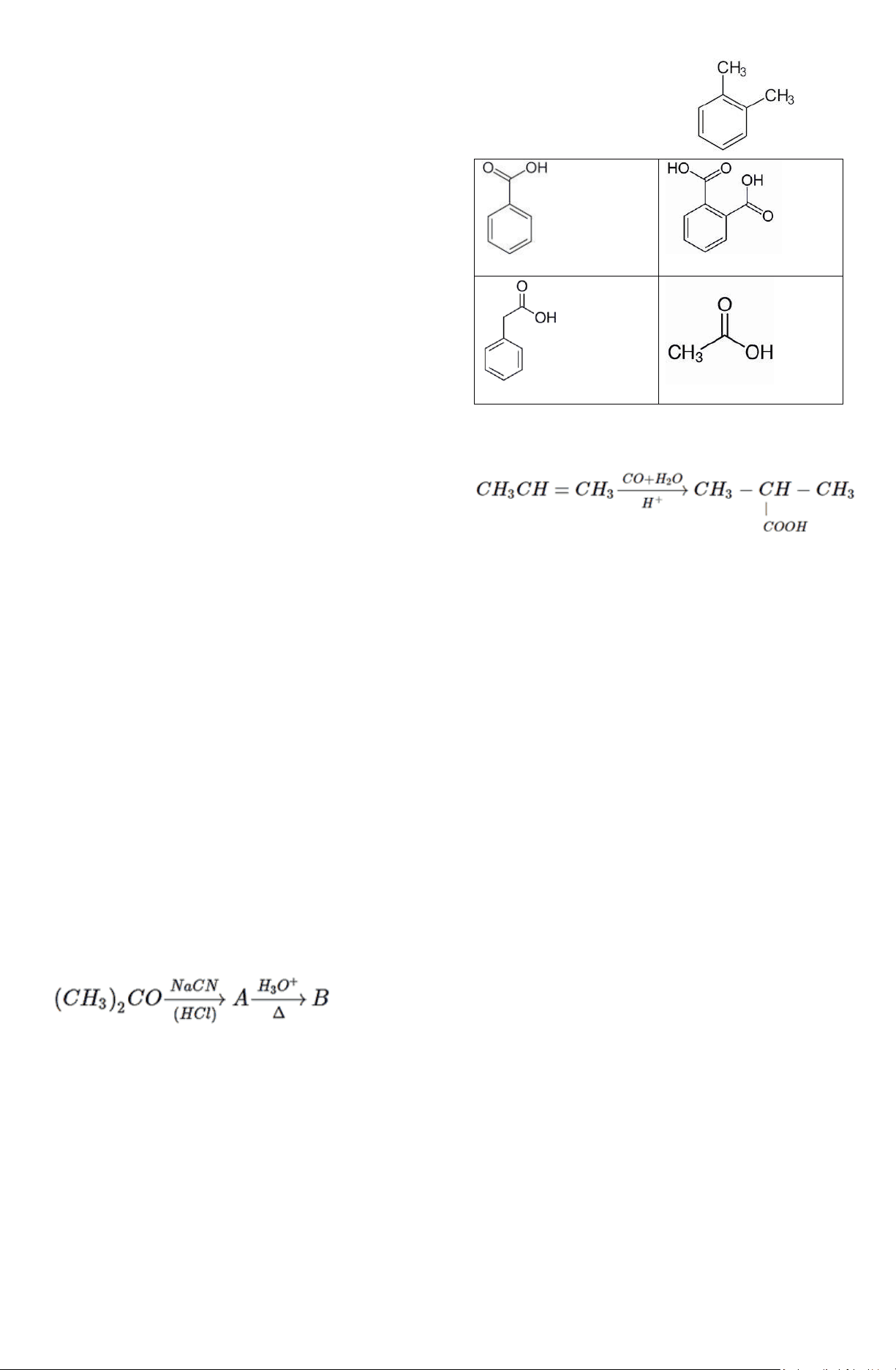
a) Benzoic acid c) Phthalic acid
b) Phenyl acetic acid d) Acetic acid
a) Wurtz reactions b) Koch reaction c) Clemenson’s reduction d) Kolbe’s reaction
2 2. In the anion HCOO− the two carbon-oxygen bonds are found to be of equal length. What is the reason for it? a) Electronic orbitals of carbon atom are hybridised
b) The C = O bond is weaker than the C – O bond c) The anion HCOO− has two resonating structures d) The anion is obtained by removal of a proton form the acid molecule
Find product yielded by acetic acid in set of given reactions What would be product C? a) CH 3 −C-C 2 H 5 (OH)C 6 H 5 b) CH 3 CH(OH)C 2 H 5 c) CH 3 COC 6 H 5 d) CH 3 CH(OH)C 6 H 5
a) Salicylic acid’s a monobasic acid b) Methyl salicylate is an ester c) Salicylic acid gives violet colour with neutral ferric chloride as well as brisk effervescence with sodium bicarbonate d) Methyl salicylate does not occur in natural oils
CH 3 OH + CO →?
a) Ethyl formate b) Methyl formate c) Ethyl acetate d) Methyl acetate
C 2 H 4 + CH 3 CO2H + 1⁄2 O 2 →?
a) Ethyl formate b) Vinyl formate c) Ethyl acetate d) Methyl acetate
c) Alcohol d) Alkene
i) ethyl methanoate ii) ethyl butanoate iii) ethyl ethanoate iv) ethyl propanoate
a) i > ii > iii > iv b) i > iii > iv > ii c) i > ii > iv > iii d) iii > i > iv > ii
a) CH 3 CONH 2 b) CH 3 CH 2 NH 2 c) C 2 H 6 d) CH 3 NHCH 3
5 7. The Hinsberg’s method is used for which of the following? a) Preparation of primary amines b) Preparation of secondary amines c) Preparation of tertiary amines d) Separation of amine mixtures